What’s Next for Corcept Therapeutics After FDA’s CRL for Relacorilant as a Treatment for Patients with Hypercortisolism
Date: December 31, 2025
Company: Corcept Therapeutics (NASDAQ: CORT)
Asset: Relacorilant (Selective GR Antagonist)
The U.S. Food and Drug Administration (FDA) issued a decisive Complete Response Letter (CRL) to Corcept Therapeutics on December 31, 2025, effectively halting the New Drug Application (NDA) for Relacorilant. The candidate, a selective Glucocorticoid receptor (GR) antagonist, was seeking approval for the treatment of Hypercortisolism (specifically hypertension secondary to the condition).
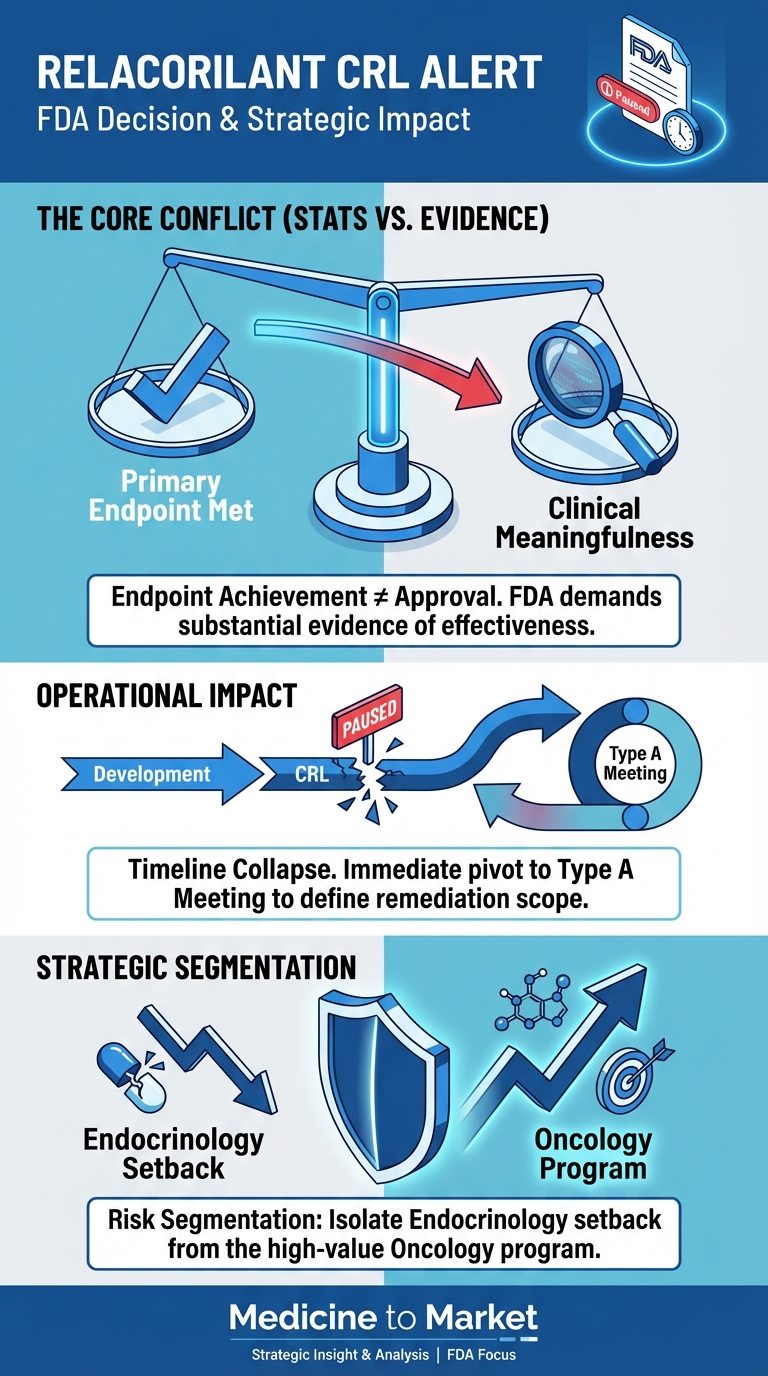
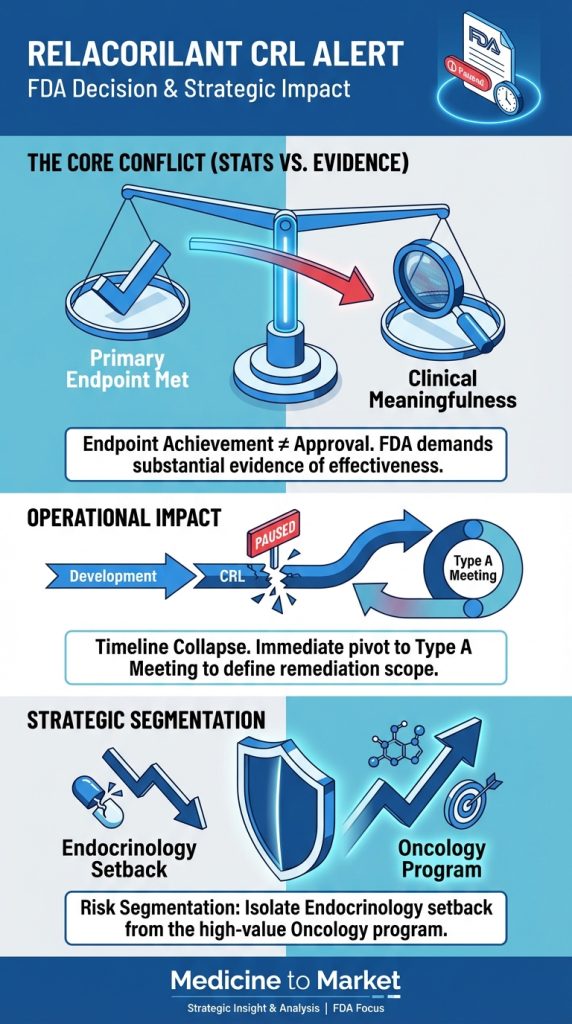
While the pivotal Phase 3 GRACE trial met its primary statistical endpoints, the Agency determined that the data package did not demonstrate a sufficiently favorable benefit-risk profile. This development represents a strategic repudiation of the current evidence package, collapsing the planned commercial launch timeline and necessitating an immediate operational pivot from the Redwood City-based biotech.
Key Takeaways for Investors and Stakeholders
-
Regulatory Action: The FDA issued a CRL, signaling that statistical significance in the GRACE trial was insufficient for approval without further evidence of clinical meaningfulness.
-
Immediate Next Step: Corcept will schedule a Type A meeting with the FDA to determine if re-analysis of data is sufficient or if a new Phase 3 trial (3-5 year delay) is required.
-
Portfolio Segmentation: Management is aggressively separating this setback from the Platinum-Resistant Ovarian Cancer program (PDUFA July 2026), which relies on distinct efficacy endpoints (OS/PFS).
-
Commercial Status: Launch preparations for the Hypercortisolism indication have ceased; reliance on the incumbent product, Korlym®, continues.
Decoding the CRL: Why “Endpoint Achievement” Failed
The core message from the FDA is a critical lesson for the pharmaceutical sector in 2026: Endpoint achievement is not synonymous with regulatory approval.
Despite the GRACE trial meeting its primary statistical measure, the Agency signaled that the “totality of evidence” was lacking. This suggests the FDA found the data qualitative lacking in critical areas, potentially including:
-
Durability of Response: Whether the therapeutic effect holds up over a chronic treatment window.
-
Clinical Meaningfulness: Whether the statistical drop in biomarkers translates to tangible quality-of-life improvements for patients.
-
Subgroup Robustness: Concerns regarding efficacy across specific patient cohorts.
CEO Joseph K. Belanoff, MD, faces the immediate challenge of proving that cortisol modulation via Relacorilant offers a substantial improvement over existing therapies like Korlym®.
The Strategic Pivot: The Type A Meeting Mandate
The company’s immediate future hinges on the upcoming Type A meeting with the FDA. This interaction will define the cost and duration of the remediation path.
Scenario A: Re-Analysis (Best Case)
If the FDA permits a complex re-analysis of existing GRACE trial data or additional subgroup stratification, the delay could be limited to 12–18 months.
Scenario B: New Phase 3 Trial (Worst Case)
If the Agency demands a new, multi-year Phase 3 study to generate “additional effectiveness evidence,” the timeline could shift by 3 to 5 years. This would require an unplanned, eight-figure capital expenditure, severely impacting the 2026-2027 financial forecast.
Operational Mandate: Executive leadership must immediately halt commercial spend on the Hypercortisolism indication and re-allocate capital to regulatory compliance and the preservation of the oncology pipeline.
The “Firewall” Strategy: Protecting the Oncology Pipeline
A critical objective for Corcept leadership is to prevent “regulatory contagion”—the market perception that the failure in endocrinology predicts failure in oncology.
The Relacorilant program for Platinum-Resistant Ovarian Cancer must be viewed through a separate lens:
-
Different Endpoints: The oncology NDA (PDUFA July 11, 2026) is judged on Progression-Free Survival (PFS) and Overall Survival (OS), which are objective measures distinct from the “clinical meaningfulness” hurdles of chronic disease management.
-
Different Need: Ovarian cancer represents a high unmet medical need with potential Orphan Drug benefits.
-
Regulatory Diversification: The oncology asset is under parallel review by the European Medicines Agency (EMA), providing geographic risk diversification.
Investors should note that the efficacy issues cited in the Hypercortisolism CRL (symptom management and quality of life) do not inherently apply to the survival data generated in the ROSELLA oncology trial.
Financial Implications and Market Outlook
The issuance of the CRL forces a “hard reset” on Corcept’s valuation models.
-
Revenue Reliance: The company remains dependent on Korlym® revenue for the foreseeable future.
-
Forecast Revision: 2026 financial guidance must be audited to remove projected Relacorilant revenue and account for remediation costs.
-
Capital Allocation: Resources previously earmarked for the commercial launch must be redirected to R&D and regulatory affairs.
Conclusion: A Mandate for Evidence Quality
The FDA’s rejection of Relacorilant for Hypercortisolism serves as a definitive signal that the regulatory bar has risen. The Agency is demanding proof that innovation translates into indisputable patient benefit.
For Corcept Therapeutics, the path forward requires rapid clarity. The Type A meeting will determine if this is a temporary delay or a fundamental restructuring of the endocrine franchise. Until then, the investment thesis relies heavily on the “Firewall Strategy”—isolating the high-value oncology catalyst from the current regulatory setback.
Frequently Asked Questions (FAQ) regarding the Relacorilant CRL
What is a Complete Response Letter (CRL)? A CRL is a communication from the FDA informing a drug sponsor that their application cannot be approved in its current form. It outlines specific deficiencies and recommends actions the applicant might take to get the application approved.
Does this CRL affect the Relacorilant Ovarian Cancer program? Technically, no. The Ovarian Cancer application relies on data from the ROSELLA trial with different endpoints (survival) compared to the Hypercortisolism application. However, market sentiment often correlates the two risks.
What is the next step for Corcept Therapeutics? Corcept will request a Type A meeting with the FDA within 30 days to clarify exactly what additional data is required to resubmit the NDA.
References
Infographic Summary