TLDR:
Crinetics Pharmaceuticals (NASDAQ: CRNX) is a leading clinical-stage biopharmaceutical company addressing unmet needs in endocrinology and endocrine-related oncology. With its flagship candidate paltusotine—an oral SST2 agonist in late-stage clinical development for acromegaly—and a diversified pipeline including therapies for carcinoid syndrome, CAH, and beyond, the company is transitioning toward commercialization. Strengthened by a $575 million public offering and a robust cash position of $1.4 billion, Crinetics is backed by strong analyst sentiment and promising near-term catalysts.
Interactive Chatbot:
For those interested in exploring the data further, we’ve developed an interactive chatbot trained on Crinetics data and insights on acromegaly. This tool provides an engaging, hands-on way to dive deeper into our analysis. Please note, the chatbot is for informational use only and does not offer medical or investment advice.
Crinetics Pharmaceuticals (NASDAQ: CRNX): Strategic Overview and Financial Analysis
Crinetics Pharmaceuticals (NASDAQ: CRNX) has emerged as a leading clinical-stage biopharmaceutical company focused on addressing unmet medical needs in endocrinology and endocrine-related oncology. With a robust pipeline of novel oral therapeutics, strategic financial maneuvers, and a disciplined approach to investor communications, Crinetics has positioned itself as a compelling investment opportunity in the rare disease space. The company’s recent New Drug Application (NDA) submission for paltusotine in acromegaly, coupled with a strengthened balance sheet through a $575 million public offering, underscores its transition toward commercialization and late-stage clinical development.
Company Overview and Strategic Positioning
Crinetics Pharmaceuticals, founded in 2008, specializes in discovering and developing small-molecule therapies targeting endocrine disorders and related tumors. The company’s core competency lies in its ability to design nonpeptide agonists and antagonists that modulate hormone receptors, offering oral alternatives to injectable standards of care. As of February 2025, Crinetics’ market capitalization stands at approximately $3.5 billion, reflecting investor confidence in its pipeline and execution capabilities.
The company’s lead candidate, paltusotine, an oral somatostatin receptor type 2 (SST2) agonist, has completed Phase 3 trials for acromegaly and Phase 2 studies for carcinoid syndrome. With an NDA submission to the FDA in September 2024, paltusotine is poised to become the first oral therapy for acromegaly, potentially disrupting a market dominated by injectable somatostatin analogs. Beyond paltusotine, Crinetics has advanced four additional candidates into IND-enabling studies, including a novel nonpeptide drug conjugate (NDC) platform targeting neuroendocrine tumors (NETs).
Financial Performance and Capital Allocation
Key Financial Metrics (Q3 2024)
- Cash, Cash Equivalents, and Investments: $862.7 million as of September 30, 2024, bolstered by an upsized $575 million public offering in October 2024, extending the cash runway into 2029.
- Research & Development (R&D) Expenses: $61.9 million for Q3 2024, up 41% year-over-year (YoY), driven by clinical trial costs and pipeline expansion.
- General & Administrative (G&A) Expenses: $25.9 million for Q3 2024, reflecting investments in pre-commercial activities for paltusotine.
- Net Loss: $76.8 million for Q3 2024, aligning with expectations for a pre-revenue biotech advancing multiple clinical programs.
Balance Sheet Highlights
- Total Assets: $937.4 million as of Q3 2024, up from $635.4 million in December 2023, supported by equity financing.
- Accumulated Deficit: $871.5 million, typical for clinical-stage companies prioritizing R&D over profitability.
Crinetics’ disciplined capital allocation is evident in its $350 million private placement in February 2024 and subsequent $575 million public offering, which minimized dilution while securing funds for paltusotine’s launch and pipeline development.
Clinical Pipeline and Development Milestones
Late-Stage Candidates
- Paltusotine (SST2 Agonist)
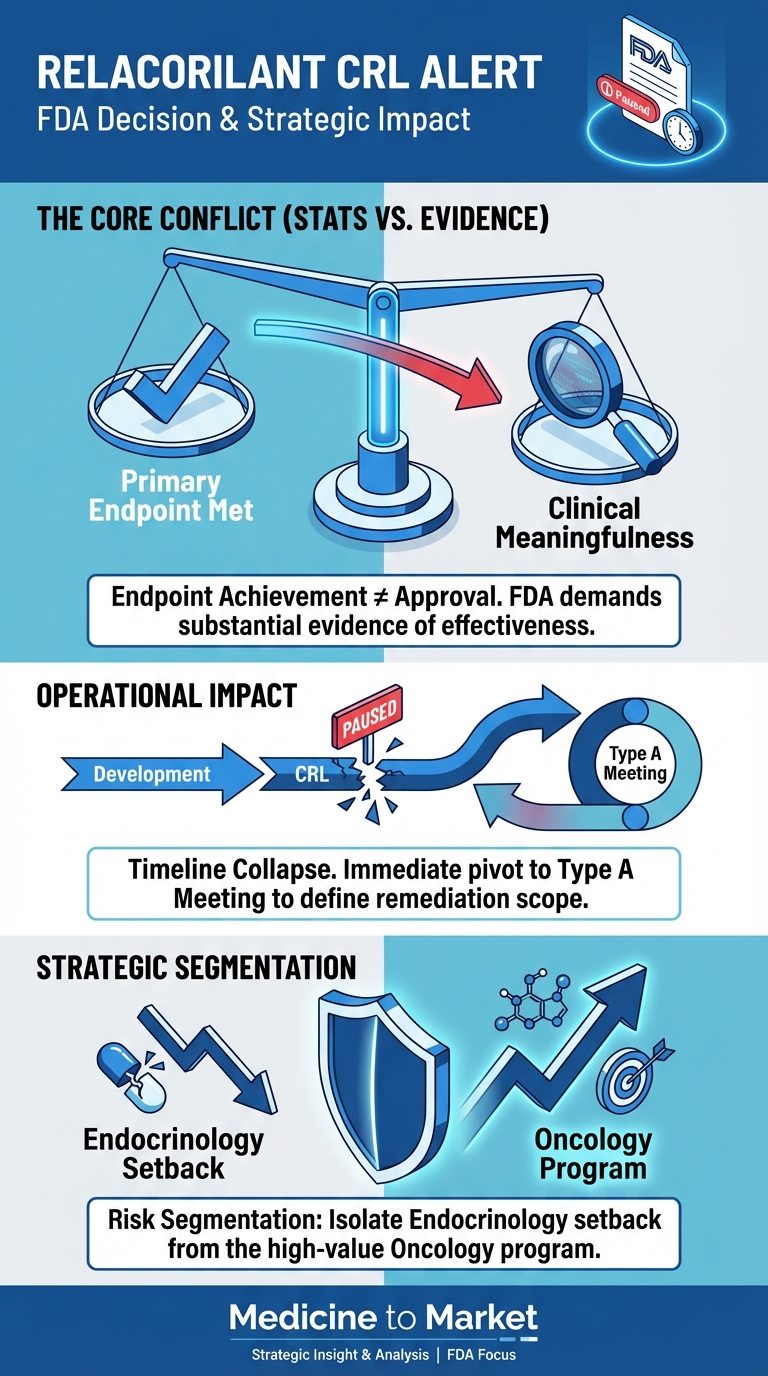
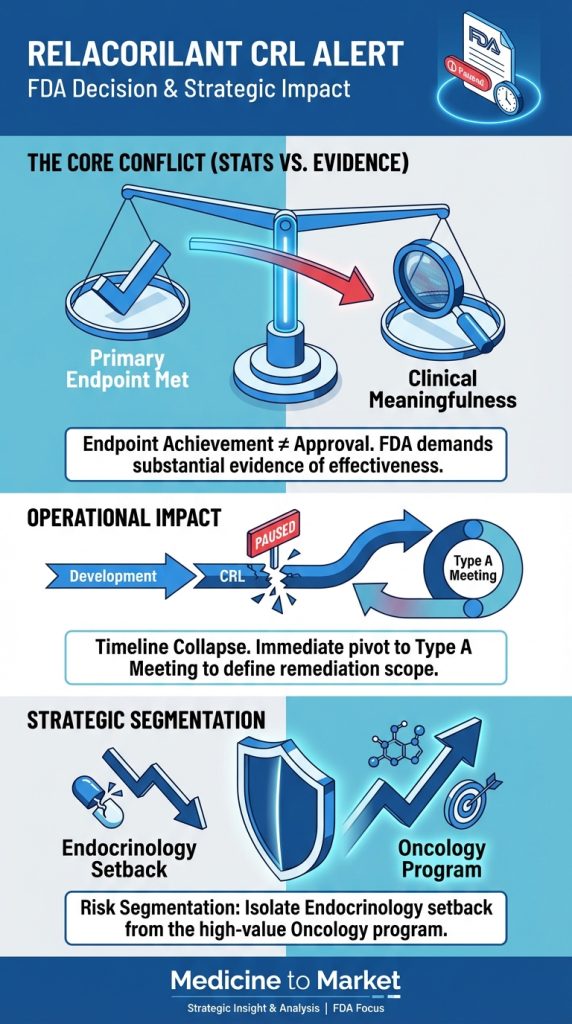
- Acromegaly: NDA submitted in September 2024; FDA decision expected in late 2025. Peak sales estimates range from $1.2–$1.8 billion, assuming approval and market penetration against injectable competitors.
- Carcinoid Syndrome: Phase 3 trial initiation planned for late 2024, targeting a $500 million+ market opportunity.
- Atumelnant (ACTH Antagonist)
- Congenital Adrenal Hyperplasia (CAH): Phase 2 data presented at ENDO 2024 showed cortisol normalization in 60% of patients.
- Cushing’s Disease: Phase 2 trials ongoing, with topline results expected in 2025.
Preclinical and Early-Stage Programs
- TSH Receptor Antagonist: IND filing planned for 2025 for Graves’ disease and thyroid eye disease.
- Nonpeptide Drug Conjugates (NDCs): First candidate (CRN09682) targets SST2-expressing tumors, with preclinical data presented at NANETS 2024.
- Oral GLP-1/GIP Agonists: Dual-targeted therapies for obesity and diabetes, leveraging Crinetics’ expertise in nonpeptide design.
Analyst Sentiment and Valuation Considerations
Crinetics is covered by 13 analysts, with a consensus “Buy” rating and a 12-month price target range of $65–$85 (current price: ~$52). Key valuation drivers include:
- Paltusotine’s Commercial Potential: Analysts project a 70% probability of approval for acromegaly, with a 2026 revenue estimate of $320 million.
- Pipeline Diversification: The NDC platform and TSH antagonist program contribute to a sum-of-parts valuation exceeding $5 billion in bullish scenarios.
- Cash Runway: $1.4 billion post-offering provides flexibility to advance four IND candidates without near-term dilution.
Risk Factors and Mitigation Strategies
- Clinical and Regulatory Risks
- Paltusotine’s approval hinges on FDA review of Phase 3 PATHFNDR data, which showed 85% of patients maintaining IGF-1 levels ≤1.0x upper limit of normal. Crinetics has engaged regulatory consultants to expedite interactions.
- Market Competition
- Novartis’ Sandostatin LAR and Ipsen’s Somatuline dominate the acromegaly market. Paltusotine’s oral administration and dosing convenience are key differentiators.
- Pipeline Setbacks
- The TSH antagonist program, while promising, faces preclinical toxicity risks. Crinetics’ platform approach mitigates single-asset dependency.
- Financial Execution
- Post-approval, Crinetics must balance commercialization costs (e.g., salesforce expansion) with R&D investments. Partnerships, like the SKK collaboration in Japan, provide non-dilutive funding.
Conclusion and Thesis
IMPORTANT: Our content NEVER contains medical or investment advice. For informational use only.
Crinetics Pharmaceuticals represents a high-conviction opportunity in the rare disease sector, combining a de-risked late-stage asset (paltusotine) with a deep preclinical pipeline. The company’s $1.4 billion cash position, disciplined capital allocation, and focus on oral therapies position it to capture significant market share in acromegaly and carcinoid syndrome while advancing next-generation endocrinology treatments. Near-term catalysts include the FDA’s NDA acceptance (Q4 2024) and Phase 3 initiation for paltusotine in carcinoid syndrome. For investors with a 3–5 year horizon, CRNX offers asymmetric upside potential as it transitions from clinical development to commercial execution.
Sources: