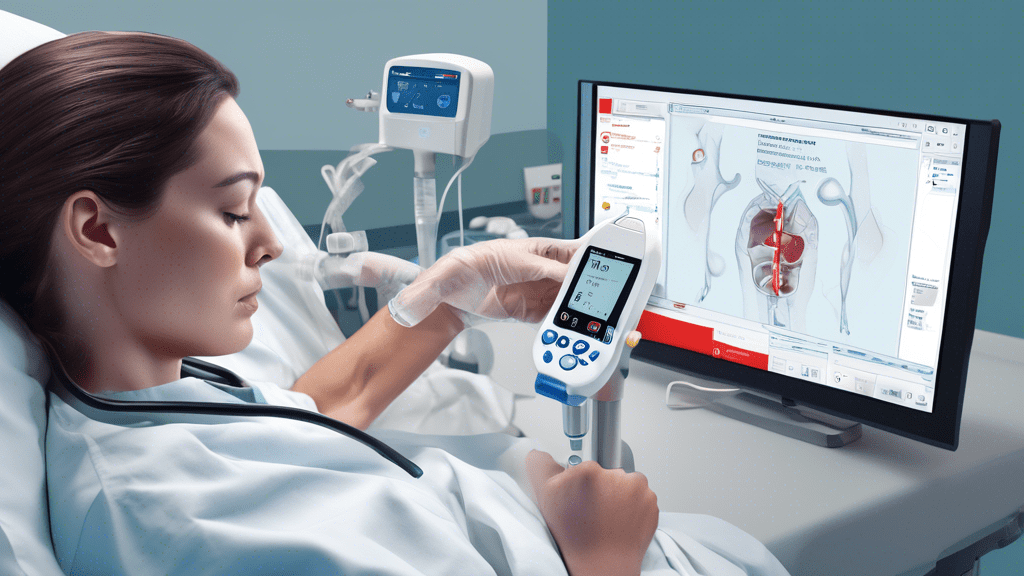
FDA Approves Abbott’s i-STAT TBI Whole Blood Rapid Test to Assess Patients for Concussions at Bedside
In a significant advancement for the management of traumatic brain injuries (TBI), the U.S. Food and Drug Administration (FDA) has granted approval for Abbott’s i-STAT TBI Whole Blood Rapid Test. This innovative test represents a major leap forward in facilitating the rapid assessment of patients for concussions directly at the point of care, such as at the bedside in hospitals or in emergency care settings.
Understanding the i-STAT TBI Whole Blood Rapid Test
The i-STAT TBI Whole Blood Rapid Test is designed to run on Abbott’s handheld i-STAT Alinity platform. This diagnostic tool works by detecting specific proteins that are present in the blood following a traumatic brain injury. By performing the test with just a few drops of blood, healthcare professionals can obtain results within minutes. This rapid turnaround is crucial for the timely management and treatment of patients suspected of having concussions, potentially improving outcomes by reducing the window between injury assessment and intervention.
Implications for Concussion Management
Concussions, a form of traumatic brain injury, can have serious implications if not promptly diagnosed and managed. Traditional methods for assessing TBIs often involve CT scans, which, while effective, can be time-consuming, costly, and expose patients to radiation. The availability of a rapid, point-of-care test like the i-STAT TBI Whole Blood Rapid Test offers a non-invasive, efficient alternative for evaluating patients with suspected concussions. This can be particularly beneficial in sports medicine, military, and emergency room settings where quick decision-making is essential.
Approval and Future Directions
The FDA’s approval of the i-STAT TBI Whole Blood Rapid Test was based on a comprehensive review of clinical trial data, which demonstrated the test’s efficacy in identifying patients with TBIs. Abbott has expressed its commitment to further research and development in the field of rapid diagnostics, with the aim of expanding the range of conditions that can be swiftly and accurately diagnosed at the point of care. This approval marks an important step not only in the management of TBIs but also in the broader effort to make healthcare more accessible and efficient.
With the i-STAT TBI Whole Blood Rapid Test, healthcare providers now have a powerful tool in their arsenal for the fight against the effects of traumatic brain injuries. As Abbott continues to innovate in the field of medical diagnostics, the potential for further enhancing patient care and treatment outcomes looks more promising than ever.