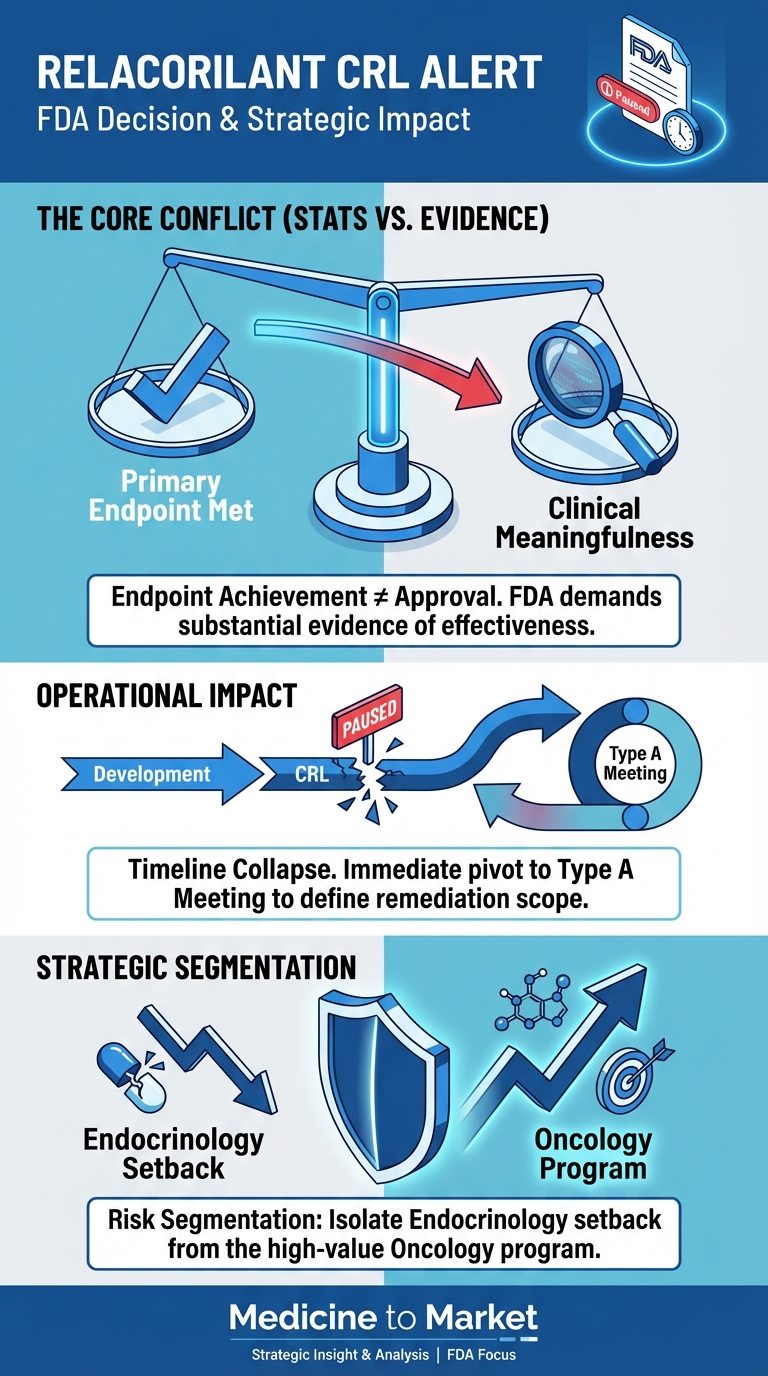
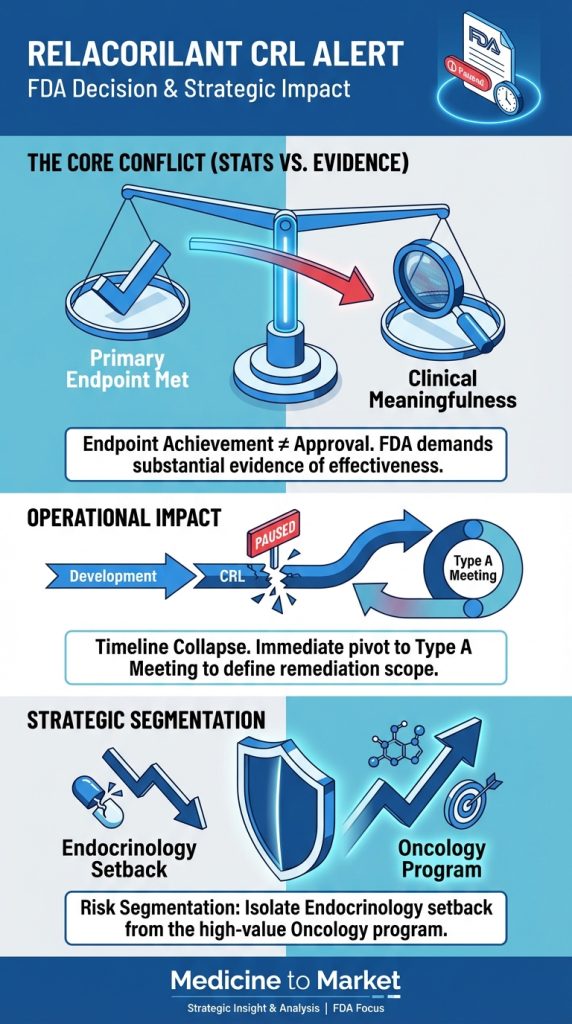
In a landmark agreement signed on September 30, 2025, Pfizer revealed its commitment to offering ‘most favored nation’ drug pricing to Medicaid programs across the United States, alongside initiatives for discounted prices accessible to consumers via a newly launched website.
This deal, emblematic of ongoing efforts to address prescription drug affordability, comes amid a backdrop of heightened scrutiny regarding pharmaceutical pricing in the U.S.
The implications of this agreement raise critical questions about its potential impact on consumers and the broader healthcare landscape moving forward.

Key Takeaways
- Pfizer’s deal introduces ‘most favored nation’ pricing for Medicaid but won’t impact those with commercial insurance.
- The agreement includes a $70 billion commitment to R&D, but its overall effect on drug prices may be limited.
- Experts warn that the deal may serve more as a public relations initiative rather than a substantial price reduction for consumers.
Understanding ‘Most Favored Nation’ Pricing
As the pharmaceutical landscape continues to evolve, executives in the biotech sector must closely examine developments like the September 30, 2025, agreement between Pfizer and the Trump administration regarding ‘most favored nation’ (MFN) drug pricing.
This agreement stipulates that state Medicaid programs receive discounts aligned with the lowest prices paid by other countries, while direct-to-consumer prices through a newly launched website aim to provide an alternative for patients.
However, it explicitly excludes those with commercial insurance and individuals choosing not to pay out-of-pocket, creating a gap that could limit its overall effectiveness.
The agreement also brings with it a three-year moratorium on tariffs related to Pfizer’s drug imports, amid a national security review, marking a significant shift in the dialogue surrounding pharmaceutical pricing.
Moreover, Pfizer’s commitment to invest $70 billion in research and development over the coming years may signal the company’s dedication to innovation; however, the lack of transparency regarding the deal’s specific terms leaves uncertainty about its true implications for U.S.
drug pricing.
Experts within the field have voiced concerns that the MFN strategy may serve as a public relations maneuver rather than a genuine effort to reduce consumer drug prices, particularly since the framework of Medicaid already provides substantial discounts through a longstanding mandatory rebate program.
This underscores a more profound challenge for the industry, as many Americans primarily obtain their medications through commercial insurance, where the impacts of such pricing initiatives may not be felt.
Moreover, with competitors like Eli Lilly and Bristol Myers Squibb exploring similar direct-to-consumer pricing pathways, the industry is witnessing a growing trend that, while innovative, is likely to encounter significant market barriers.
Notably, high-cost medications, particularly those essential for oncology and rare diseases, remain largely insulated from these programs, raising questions about accessibility for the most vulnerable patients.
The call for a balanced pricing approach that recognizes both innovation and sustainability may provide a narrative framework for discussions; however, without clear guidelines and outcomes, the MFN agreement’s long-term viability remains questionable in reshaping the U.S.
pharmaceutical pricing paradigm.
Implications of the Deal for Medicaid and Consumers
As this agreement unfolds, analysts suggest that the implications for Medicaid and consumers remain complex and multifaceted.
Medicaid’s existing rebate structure could complicate the intended benefits of the MFN pricing strategy, as it has historically allowed for substantial discounts.
Additionally, since the new pricing model targets only select demographics, the overall reach may be limited, reducing its impact on healthcare costs for the majority of consumers.
The reaction from advocacy groups has been cautious, indicating that the changes might not translate into significant cost savings or improved access to medications for those reliant on commercial insurance or who are unable to afford out-of-pocket expenses.
Institutions like the Kaiser Family Foundation are closely monitoring the situation, emphasizing that any perceived benefits must be weighed against the reality that many patients could still face exorbitant drug prices despite the agreement.
Executives in the biotech space may find it critical to engage with these developments to understand how such agreements could influence market dynamics and patient access moving forward.